Ci-DMS – Product Overview
Fully featured MES for pharmaceutical weigh and dispense
Ci-DMS has been used by pharmaceutical manufacturers for over 20 years to streamline the move to electronic batch records and ensure compliance with GMP across their weighing and dispensing operations.
It provides full control of the weigh, dispense, addition, discharge, yield reconciliation and cleaning processes, as well as recipe management. It includes powerful reporting and analysis. It can work stand-alone or be readily integrated to ERP and other MES systems, as well as weighing equipment, barcode scanners and label printers.
Back to Solution Overview >From initial configuration through day to day operations and supervision, it provides the most intuitive user experience and richest functionality of any pharmaceutical weigh and dispense system.
Contact Us >CI-DMS functional overview
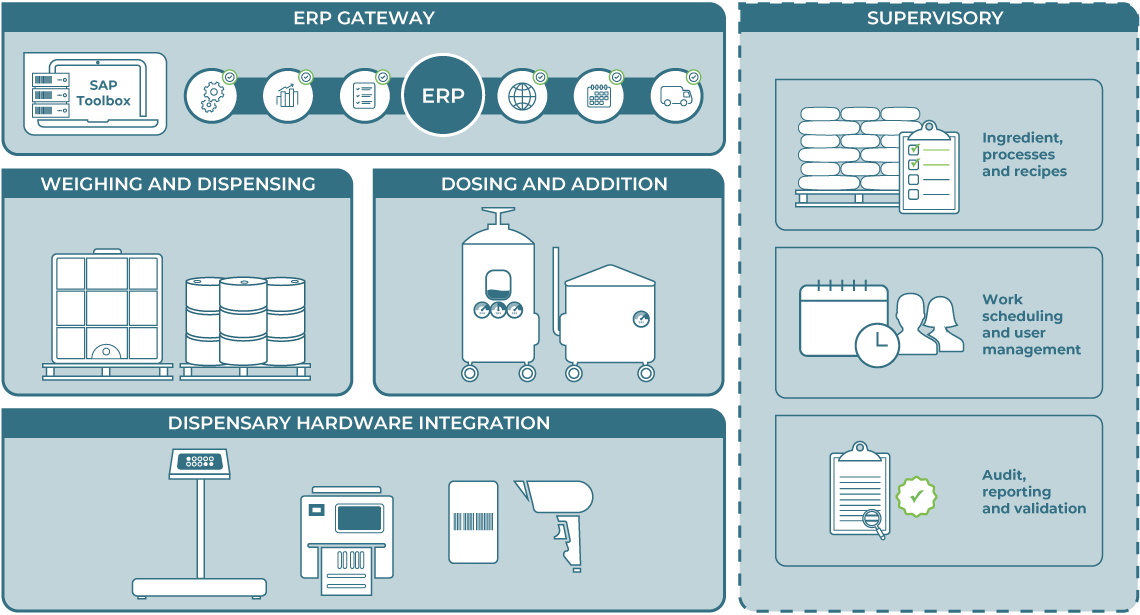
Weighing and dispensing
Ci-DMS controls and records all aspects of your manual weighing and dispensing. Operators are guided through the steps required for each recipe, including cleaning and device validation.
The system displays clear instructions and ensures any health and safety notices are acknowledged. It verifies that the right ingredients have been weighed out in the correct quantities and logs every significant action and all electronic signatures.
Ci-DMS avoids the need for manual adjustments and reckoning: it can track across multiple containers and lots and automatically adjust for potency.

Every detail adds up to more efficient, error-free weighing and dispensing
![]()
Barcode scanning to validate ingredients and lots
![]()
Integration to balances to ensure correct weighing
![]()
Ability to track across multiple containers and lots
![]()
Option to automatically adjust weights for potency
![]()
Automated label printing for dispensed and returned ingredients
![]()
Tracking stock onto pallets, drums and IBCs
![]()
Electronic approvals for key actions, checks and exceptions
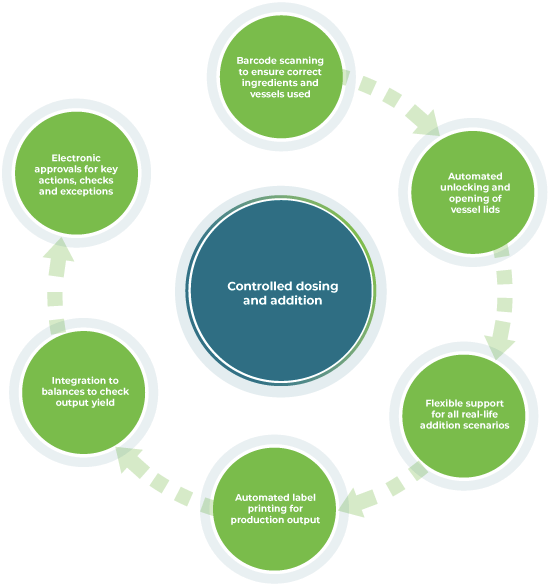
Dosing and addition
Ci-DMS guides operators through all the steps required to add raw materials into fixed and movable production vessels, including cleaning and device validation. It can run on handheld devices, providing clear instructions and health and safety notices as needed.
Pre-scanning and verification
It allows operators to pre-scan all ingredients to ensure everything is correct, including QC statuses and expiry dates. It verifies that the correct vessel has been selected and can be configured to automatically unlock and open lids.
Manual discharge management
Ci-DMS can also manage all the steps for manually discharging out of production vessels, including check weighing the output yield.
ERP integration
Ci-DMS can be tightly integrated with your ERP system without any custom coding. It includes a highly configurable interfacing module which provides comprehensive, real time data interchange with almost any ERP solution. For SAP users we also provide a pre-made set of workbench objects and documentation to further facilitate integration.
Real-Time Updates and Monitoring
Your ERP is updated with production and stock information in real time, so you can monitor any exceptions or issues without delay. Ci-DMS can also take instructions from your ERP, as high level product orders or detailed work instructions and BOM.
Functionality
- All processes maintained under version control
- Full control over the weigh, dispense, addition, discharge, yield reconciliation and cleaning processes
- Electronic batch execution and batch records
- Powerful reporting and analysis facilities
Interfaces
- Web services interface for software integration
- OPC, ethernet and RS232 interfaces for hardware integration
Regulatory
- Traceable and auditable
- GAMP Category 4 – configured software
- Designed and validated to GAMP 5 standards
- Developed according to their principles of ISA 88/95
- Designed to support adherence to the regulatory data integrity guidelines
Implementation
- Modular and scalable
- Supports a phased implementation
- Quick to implement, configure and validate
- Intuitive design enabling rapid operator take-up
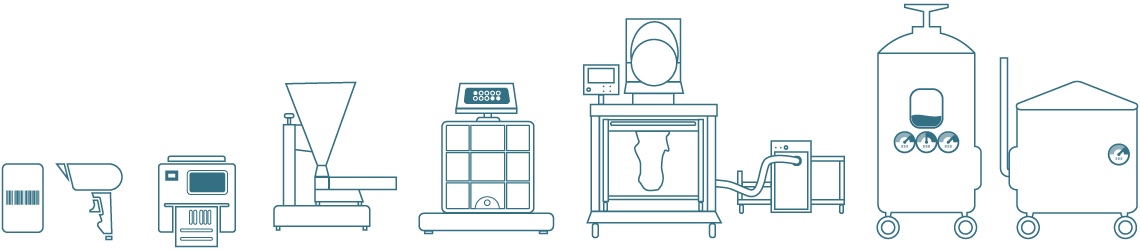
Dispensary hardware integration
To maximise your productivity gains and avoid costly manual checking, Ci-DMS can integrate to the widest range of hardware, from barcode scanners, scales and printers through to sophisticated equipment such as loss-in-weight feeders, material transfer systems and process vessels.
With these integrations you can include equipment validation within Ci-DMS processes, as well as managing alerts, performing diagnostics and ensuring that cleaning routines are followed.
Managing ingredients, processes and recipes
Ci-DMS provides highly configurable and flexible management of all your ingredients, processes and recipes. This functionality allows it to operate fully stand-alone or you may choose to delegate some or all of these capabilities to your ERP system.
As part of each process you can define any additional instructions that need to be shown, along with any health and safety warnings which must be shown and acknowledged. You can stipulate electronic signatures and different levels of approvals wherever required. Cleaning routines may be defined according to the sequence of ingredients, elapsed time or other criteria.
Strict recipe control
Recipes (including their ingredients and processes) are managed under strict control, ensuring appropriate approvals and preventing any changes once approved. Authorised users may suspend and archive recipes, while all activities are recorded in an audit log.
Full management of all recipes for dispensing and dosing
Display of health and safety warnings
Flexible, contextual cleaning regimes
Ability to split tasks over shifts, especially cleaning
Sophisticated configuration of electronic signatures and approvals
Scheduling work and managing users
Whether it is operating stand-alone or integrated to your ERP, Ci-DMS provides tools for scheduling and allocating jobs to specific dispensing booths and operators. It allows supervisors to monitor the progress of work and to make changes to the work queues if required.
User and role management
The system provides flexible management of users and roles which are integrated with the configuration of electronic signature and approvals. All users and logins may be fully integrated with your existing Windows user management.
- Flexible work allocation tools
- Job list and BOM from either Ci-DMS or ERP
- Live process reporting for supervisors and through ERP integration
- Integration to Windows user authentication
- Sophisticated user and role management
- Full audit trail
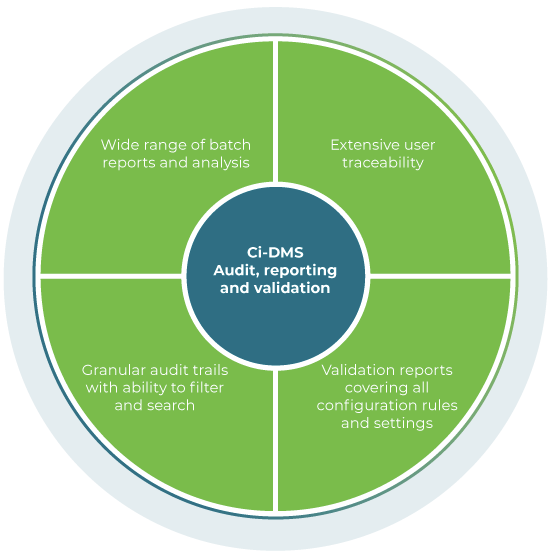
Audit, reporting and validation
Ci-DMS has been designed specifically for pharmaceutical manufacturing and includes extensive audit capabilities and a wide range of reports to cover all aspects of GMP. These support day to day operations, detailed analysis and efficient provision of regulatory information.
To streamline project delivery, Ci-DMS can generate reports with details of all the configurations and settings against which the system is validated.
Connect with Us
Discover how Ci-DMS can streamline your manual weighing and dispensing processes.