As controlled release, implantable dosage forms become more prevalent in medicine and as part of pharmaceutical companies’ portfolios, there is now more focus on how they are manufactured. What might once have been a fairly niche operation is now subject to the same scrutiny in terms of efficiency, compliance and recordkeeping, by both management and regulators.
Individual weighing has always been a key part of quality checking implantable doses. This is both to protect patients against overdoses of often highly potent treatments, and to ensure the consistency of what may be an entire course of treatment. Statistical measures of quality are not sufficient in this environment; every single dose must conform to the tight specifications. However, manual weighing is problematic in numerous respects, including cost and efficiency, risk of human error and quality of record-keeping.
At CI Precision we have worked with pharmaceutical companies for over 50 years, providing fast, efficient precision weighing solutions. Here are the challenges that we are helping them to overcome with depots, polymer implants and other implantable, controlled release, solid dose drugs:
Minimising manual labour and eliminating second checking
Manual weighing is laborious and error prone. To ensure good manufacturing practice there have to be rigorous procedures in place for each weighing operation. Depending on the product this may have to include second checking or even double weighing of each batch.
This results in disproportionately high labour costs, even before you factor in the cost of training staff in these demanding procedures.
By automating your weight sorting, you can reduce the time and labour of sorting a batch by several orders of magnitude. Operating an automatic weight sorter also requires far less specialist training and does not need a second checker.
Removing the risk from manual weighing
It is very hard for people to repeat the same activity over and over again without making the occasional mistake. Even with carefully designed procedures, errors do inevitably occur.
With implantable drugs, which are both long-lasting and often very potent, a single error could have grave consequences for the patient and the reputation of the manufacturer.
By replacing manual weight sorting with an automated system, you can remove almost all the risk of human error. An automated system will not only have highly precise weighing capabilities but also dependable sorting mechanisms and independent verification that defective doses have been correctly separated out.
Removing the bottleneck from weight checking
From an operational perspective, one of the biggest problems with manual weight checking is the amount of time it takes to process an entire batch. Even with several people working in parallel, manual weight sorting will always be the bottleneck which holds up production.
Automated weight sorters can check individual doses at far higher speeds than manual operations could ever achieve, without compromising precision or repeatability. By swapping out manual weighing stations for automated machines, manufacturers are achieving immediate improvements in output.
A better way of working in sterile conditions
Manufacturing in sterile conditions is essential for drugs that will be implanted into the human body. However, it is particularly difficult to maintain a sterile atmosphere where people are constantly in close proximity to the product, as with manual weight sorting.
Pharmaceutical manufacturers have devised effective ways of maintaining clean air flows and avoiding other sources of contamination. However, this usually adds more burden on human operators to work in very particular ways, which slows them down and increases the risk of nonconformity.
With automated weighing machines, operators do not come into such close contact with the product. Machines like the DC100 can process several hundred doses without intervention and are operated at a distance. They can be topped up without the operator coming within more than an arm’s length.
Ensuring recordkeeping is fully compliant with ALCOA+
A substantial drawback with manual weight checking is the incomplete or even questionable nature of the recordkeeping. As regulators are increasingly demanding ALCOA+ principles to be applied throughout manufacture, the weight of every dose needs to be recorded at source, in a way that isn’t subject to error and that cannot be altered.
When a facility is audited, it can also be hugely time-consuming pulling together the separate manual weighing records that are needed for review.
When you automate, every weighing is captured as an indelible record, along with confirmation that nonconformant doses have been separated out. This data can be securely shared and stored on your centralised systems.
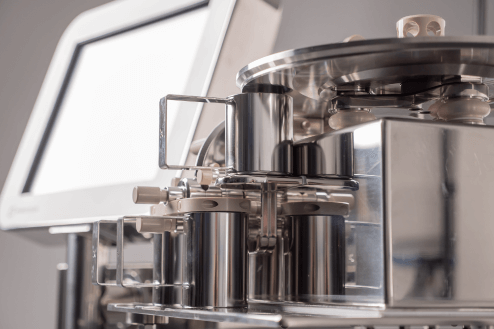
XCEL DC100: Automated, 100% weight checking of every manufactured dose
The XCEL DC100 is part of a family of high precision, high speed, automated weighing machines with a thirty year track record in the pharmaceutical sector. It is optimised for weighing implant doses and can check every item that is manufactured, automatically rejecting any which fall outside the acceptable weight.
It is a direct replacement for manual weighing, so you can easily implement it in almost any environment.
The DC100 was originally developed for a major pharmaceutical company and has since been released as a fully validated, off-the-shelf product.
Unlike conventional checkweighers, the DC100 weigh sorter can weigh a wide variety of implant types and sizes to very high accuracy, without the need for different change parts. All contact parts are of high specification materials and can be swapped out easily for cleaning.
Connect with Us
Contact the CI Precision team today and book your individual project discovery session.